Epistemic status: I have no expertise, and news about COVID-19 changes by the minute so this is likely somewhat outdated, but below is my best guess from what I have read so far (h/t Tyler Cowen, Alex Tabarrok, Eric Topol, and many others).
- Overview
- Prior research
- mRNA technology
- Genome sequencing
- Development
- Manufacturing
- Distribution
- Endnotes
Overview
We wanted flying cars… instead we got >90% effective vaccines within 10 months of a novel viral pandemic!1
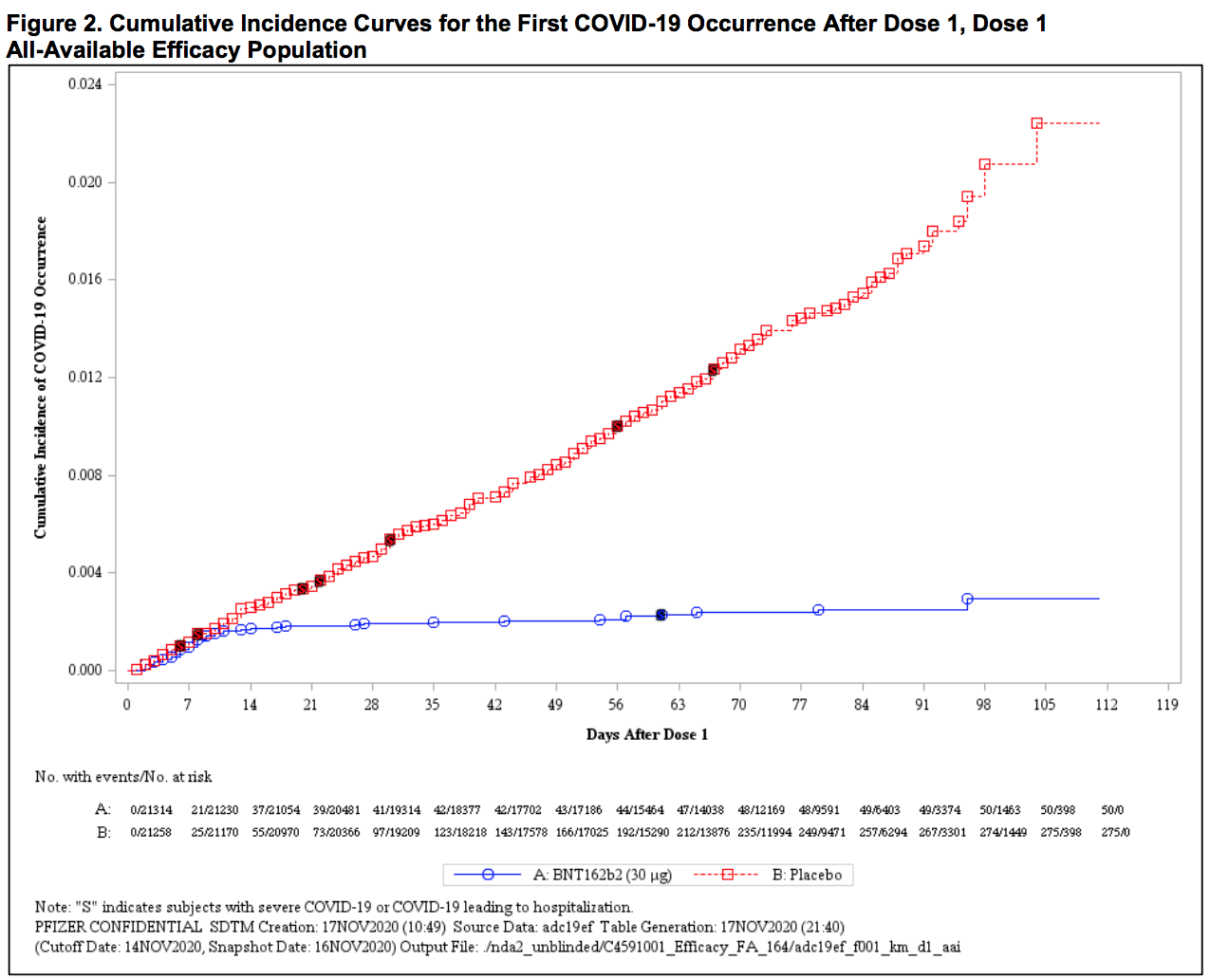 This is a triumph (blue: vaccine; red: placebo).2
This is a triumph (blue: vaccine; red: placebo).2
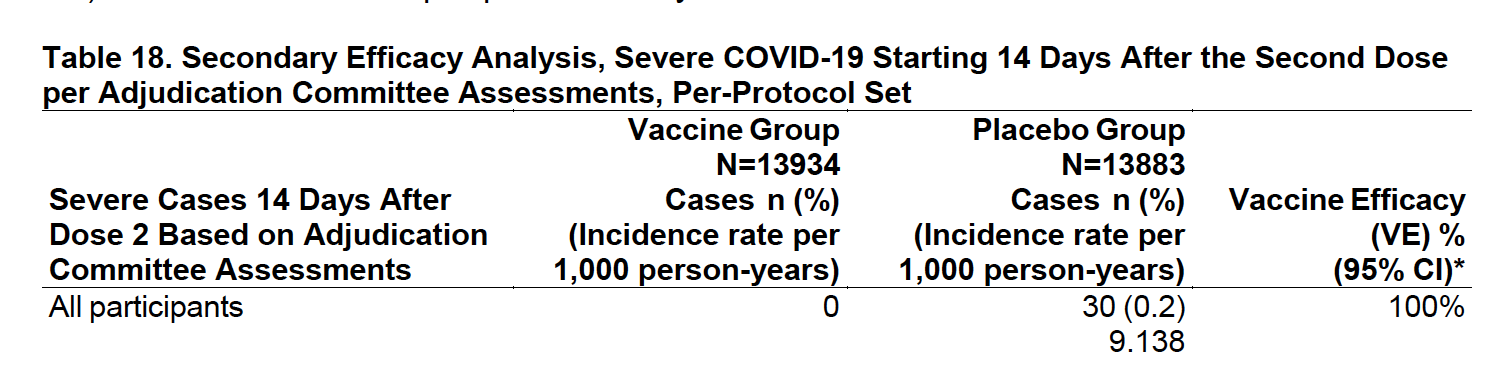
Pfizer and BioNTech published their phase 3 analysis of their messenger RNA (mRNA) candidate and reports it to be 95% effective, with 162 infection cases in the placebo group versus 8 in the vaccine group in the 43,548-patient trial. Interestingly, efficacy against severe COVID-19 occurring after the first dose was 88.9%! The vaccine is well tolerated and there are minimal (<1.0%) severe side-effects (a.k.a. grade 3 adverse events) including fatigue (3.8%) and headache (2.0%). The most commonly transient side-effects that resolve shortly are fatigue and headache (59% and 52%, respectively, after the second dose, among younger vaccine recipients; 51% and 39% among older recipients). Funny enough, fatigue and headache were also reported by many placebo recipients (23% and 24%, respectively, after the second dose, among younger vaccine recipients; 17% and 14% among older recipients). Overall, safety over a median of 2 months was similar to that of other viral vaccines. Unless you are a 90-year-old grandma or William Shakespeare, we might already know the “long-term side effects” by the time you get vaccinated!
The Pfizer vaccine will require 2 doses 21 days apart and cannot be removed from -70°C more than 4 times, which poses a massive distribution challenge. Nonetheless, I cannot wait to be vaccinated because vaccination is the only viable path to herd immunity.
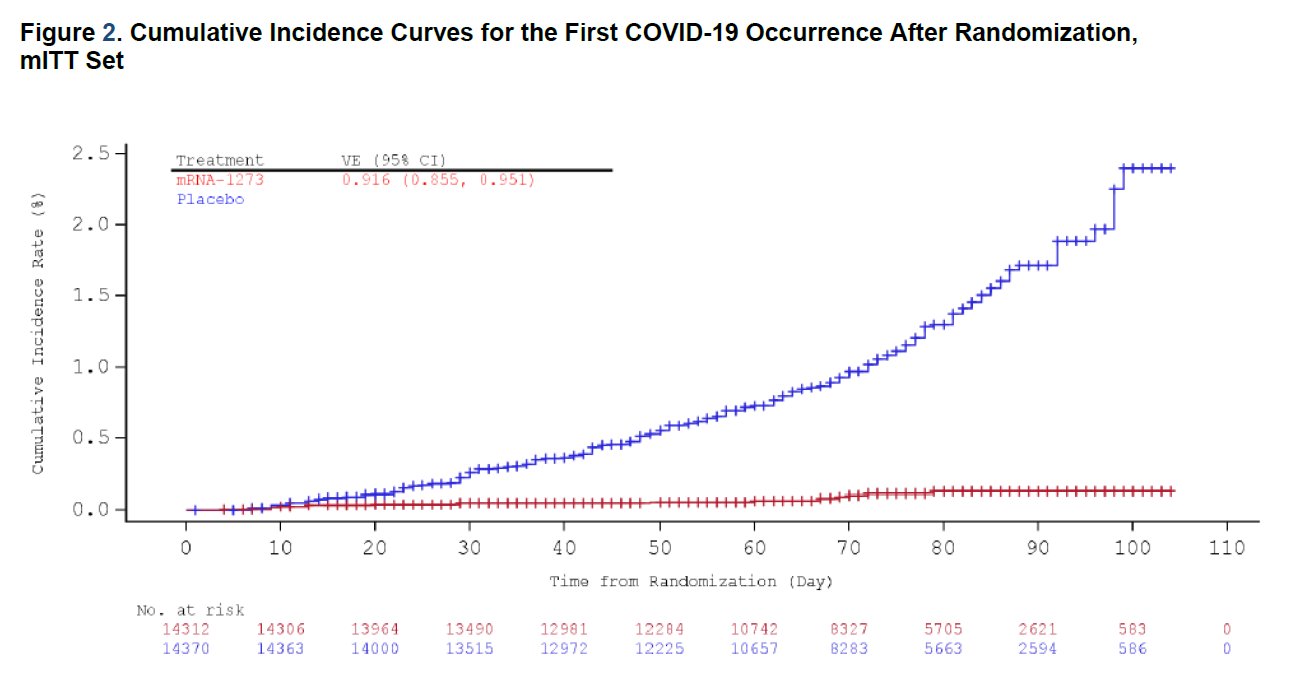 This is also extraordinary (red: vaccine; blue: placebo).
This is also extraordinary (red: vaccine; blue: placebo).
Moderna reports in their first interim analysis of their phase 3 trial that their own mRNA candidate is 94.5% effective, with 90 infection cases in the placebo group versus 5 cases in the vaccine group in the 30,000-patient trial. The vaccine is generally well tolerated, with short-lived grade 3 adverse events such as fatigue (9.7%), myalgia (8.9%), arthralgia (5.2%), headache (4.5%), and pain (4.1%). It will also require 2 doses, 4 weeks apart. Also, there is some data that supports efficacy against asymptomatic infection. Most groundbreaking is that their vaccine remains stable up to 6 months under standard freezer condition (-20°C), up to 30 days under normal fridge condition (4°C), and up to 12 hours in room temperature, which means significantly easier distribution and storage with the existing infrastructure.
The Oxford-AstraZeneca viral vector3 (weakened chimpanzee adenovirus with DNA modified to force host cells to express the D614 variant SARS-CoV-2 spike protein, a virus-specific protein that gives it the crown-like appearance) vaccine was in the lead before its trials were briefly suspended when two patients got ill. Regulators were not happy because AstraZeneca did not disclose the halt before the news broke. Interim analysis of the data from its phase 2/3 trial and a parallel phase 3 trial found one dosing regimen (n=2,741) to show efficacy of 90% when the vaccine was given as a half dose, followed by a full dose at least one month apart, and another dosing regimen (n=8,895) showed 62% efficacy when given as two full doses at least one month apart. We still have no idea why a first full dose has lower efficacy than a first half dose, but a wild guess would be that the two-full-dose regime raised too many antibodies to the adenovirus vector itself. There is some data that support efficacy against asymptomatic infection. There is no information on side effects, but there are hospitalisations or severe infection cases in participants. The vaccine can be stored and transported at normal refrigerated conditions (2-8 °C) for at least six months.
Apparently, the half-dose-then-full-dose protocol was an accident!? In April, when Oxford contracted Italy’s IRBM/Advent to complement their vaccine production, the Italian manufacturer quality-checked its vaccine batch using standard quantitative PCR (qPCR), but Oxford researchers used spectrophotometry (measures how much UV light viral matter absorbs) which is notoriously imprecise. Alas, polysorbate 80, a common emulsifier used in vaccines to facilitate mixing, had interfered with the UV-light meter that, resulting in the overestimation of the concentration of viral matter. The Oxford researchers trusted their own result so they used the lower-strength dosage. Soon, the researchers noticed milder side effects than expected4. These blunders do not help with confidence in the vaccines.
 Let us hope Oxford-AstraZeneca pulls through because the world is depending on them.
Let us hope Oxford-AstraZeneca pulls through because the world is depending on them.
Meanwhile in Russia (now with more evidence and the usual caveats), the Sputnik V viral vector (modified human adenovirus) vaccine has shown 91.4% effectiveness in its 40000-patient phase 3 trial.
The Chinese Sinovac Biotech vaccine was reported to elicit quick immune response and is at least found to be safe in its Brazil trial. Note that there has yet to be any published data from its phase 2 and 3 trials in the United Arab Emirates (UAE). Also, the CEO of Sinovac has been bribing China’s drug regulators for vaccine approvals since 2003. Meanwhile, UAE has approved Sinopharm’s vaccine, claiming an efficacy of 86%, yet again without any published data. All of that has not stopped a reported 60000 people to receive it in emergency-authorised use though, including the Chinese military and to some employees of Chinese state-owned enterprises. In fact, China is aiming to vaccinate 50 million people before Chinese New Year with the Sinovac and Sinopharm candidates that still have no data. China has a safe vaccine, just not for you!
It is important to note that many of these results were only published in bare-bones press releases, and there is no information about whether asymptomatic infection is also protected, nor whether severity of symptoms is reduced. On the other hand, all other candidates, including around 10 currently in phase 3 trials, are designed to block SARS-CoV-2’s spike protein, so it is quite likely that the aforementioned candidates will not be the only ones that prove efficacious. The expected value of delaying getting sick just went way up! So stay home and stay safe.
We will only find out how long immunity from the vaccines will last by waiting and watching, and the pandemic is just barely a year old so we do not have long term data on natural immune response. That said, a paper found that memory B cells specific to spike protein were more abundant at 6 months than at 1 month, while SARS-CoV-2-specific CD4+ T cells and CD8+ T cells declined with a half-life of 3-5 months. Another paper found that IgA and IgM antibodies were short-lived (seronegative by 51 and 47 days post-symptoms), while IgG antibodies lasted longer (at least 75 days post-symptoms). Yet another paper found that functional SARS-CoV-2-specific T-cell responses are retained for at least six months. The literature paints a consistent picture of a strong, normal, lasting immune response in the vast majority of patients. Also, early vaccine data suggests antibody responses at least as strong as those found in naturally infected cases. Hence, there is (so far) every reason to think that vaccine-based immunity will be as good or better than that conferred by an actual infection.
With regards to viral mutation, a Pfizer paper found that many of the common mutations are as susceptible to antibodies raised by their vaccine. The SARS-CoV-2 G614 variant became the dominant strain (more fit than the D614), which is fortunately now well characterised and found to be responsible to the D614 spike protein vaccines. Also, SARS-COV-2 does not have the re-assortment mechanism that allows influenza virus to change so easily and quickly that warrants annual vaccination. There is an effort to monitor developing mutations so that such issues can be dealt with swiftly.
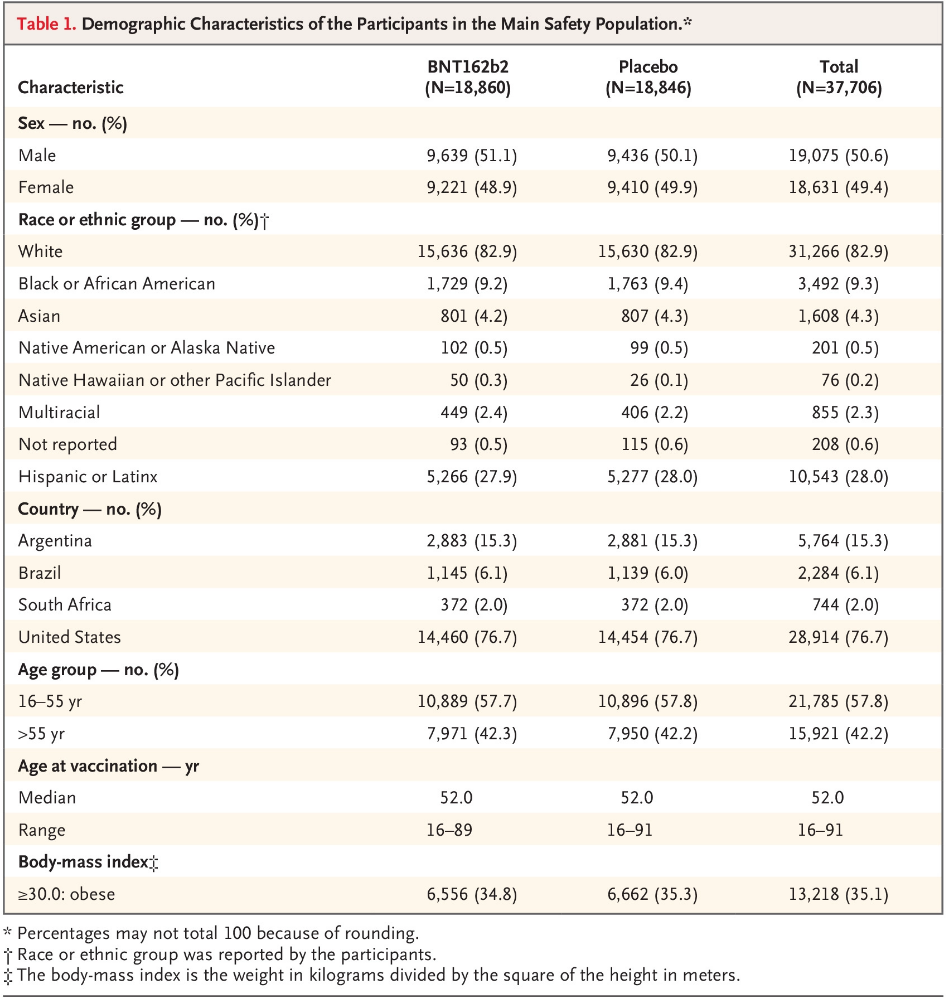
Another worry is efficacy in different demographics, but fortunately the Pfizer vaccine demonstrated 94% efficacy in patients 65 and older. Also, 42% of global participants and 30% of U.S. participants have racially and ethnically diverse backgrounds.
It is also important to note that a lot of people who get the vaccine will die or get diagnosed with serious illnesses and it will have nothing to do with the vaccines. For example, if we vaccinate 10 million people in the US today, 300 of them will die the very next day. Over the following 2 months, 4025 of them will have a heart attack, 3975 of them will have a stroke, and 14,000 will die. Hence, you should vaccinate yourself against misinformation by comparing “side effects” with the baseline statistics.
Prior research
While there are about 200 candidates globally, the Pfizer and Moderna vaccines are the only ones that rely on mRNA, a completely novel technology that was a scientific backwater before it was a multibillion-dollar idea. In fact, we did not even know mRNA existed 60 years ago. It was first proposed to exist by Jacob and Monod and was isolated soon after in 1961.
The concept of mRNA therapeutics was first demonstrated to work in mice in 1990 when injected mRNA led to detectable protein production, and a subsequent paper showed that administration of mRNA elicited physiological response in rats. However, these early promising results did not lead to substantial investment, and no mRNA vaccine nor drug has ever been approved. (That is about to change!) mRNA is notoriously unstable as free RNA breaks down quickly, posing a significant in vivo (in live cell) delivery challenge. mRNA injected into the body might also elicit unintended immune response, which is the stumbling block that led to most of the rejections of funding.
For Katalin Karikó, a Hungarian-born biochemist, mRNA was a career dead-end. Her grant applications were all rejected, and she was even demoted from her path to full professorship. When Karikó was working in the University of Pennsylvania’s neurosurgery department trying to turn RNA into stroke therapeutics, she stumbled onto Drew Weisman, now a professor of medicine at the university, who asked her to make some RNA for an HIV vaccine idea he was pursuing. After more than a decade of trial and error, they discovered in 2005 that by modifying a nucleoside (building block of mRNA), mRNA can penetrate its target cells without eliciting an immune response. This key discovery did not go unnoticed by the future founders of Moderna and BioNTech.
Derrick Rossi was a Canadian stem cell biology postdoctoral fellow at Stanford when he first came across Karikó and Weisman’s work . With another postdoctoral fellow, they discovered that modified mRNA can reprogram adult cells to act like embryonic stem cells that have the potential to develop into any cell. Embryonic stem cells are the holy grail of regenerative medicine, and Rossi’s discovery can completely sidestep the ethical controversy of harvesting them from discarded embryos.
Rossi, who is now a professor at Harvard Medical School, informed his colleague and biotech entrepreneur Timothy Springer, and the two contacted Robert Langer, a prolific inventor and legendary biochemical engineering professor at MIT. Rossi then presented his findings to Noubar Afeyan, a Lebanese immigrant and founder of Flagship Ventures. Within a several months, Rossi, Langer, Afeyan, and another physician-researcher found Moderna5, a pun on modified RNA.
In Germany, husband and wife Uğur Şahin and Özlem Türeci, both Turkish immigrant and immunology physicians-scientists, co-founded BioNTech as a spin-off of another biotech startup they previously co-founded. Like Moderna, BioNTech licensed technology developed by Karikó and Weissman. In fact, BioNTech hired Karikó in 2013 when UPenn laughed that BioNTech did not even have a website and refused to reinstate her to the faculty position she had been demoted from in 1995. When Şahin read about the COVID-19 in China in January, he realised BioNTech required a strong partner to manufacture the vaccine and reached out to the pharmaceutical giant Pfizer, which BioNTech worked with before to try to develop mRNA influenza vaccines.
Another key line of research is stablising the spike protein via 2 proline (P) substitution, without which the mRNA vaccines would have failed. In 2015, 3 years after the initial outbreak of middle East respiratory syndrome (MERS), Barney Graham, deputy director of the Vaccine Research Center (founded by Anthony Fauci at the National Institutes of Health/NIH to combat HIV), Jason McLellan, a structural biologist at the University of Texas, and Andrew Ward, a structural biologist at the Scripps Research Institute realised that if they can stabilise the spike protein for HKU1 (a coronavirus that causes the common cold, they can do the same for other deadlier coronaviruses. Nianshuang Wang, a research fellow at McLellan’s lab then, now at Regeneron Pharmaceuticals, spent 2 painstaking years trying hundreds of genetic mutations, and finally found that introducing 2P’s at the beginning of the central helix of the spike protein stabilises its confirmation upon cell entry. They had to spend another painful year facing 5 journal rejections before it was published in 2017.
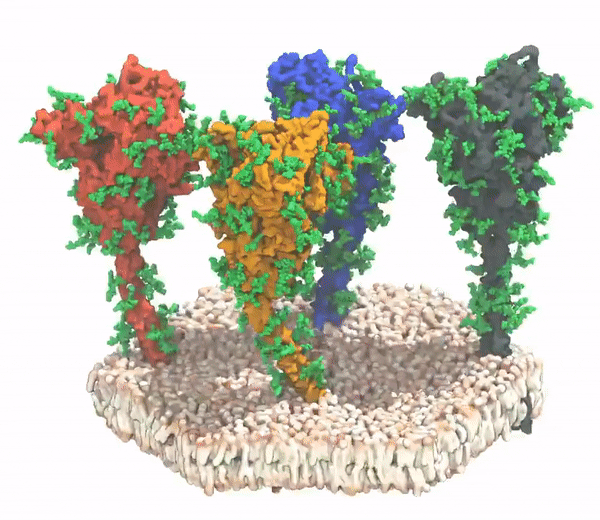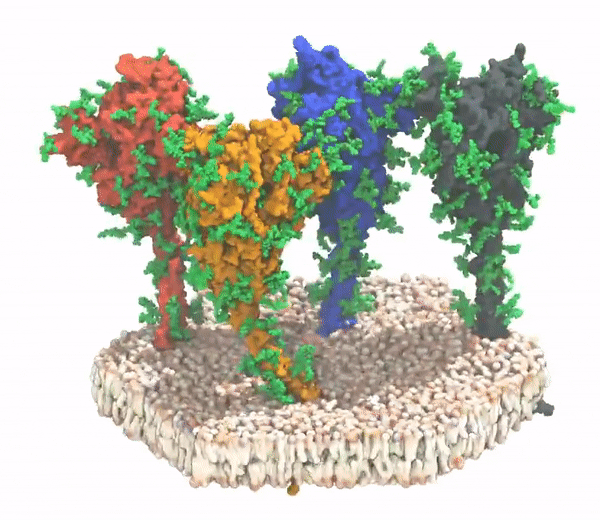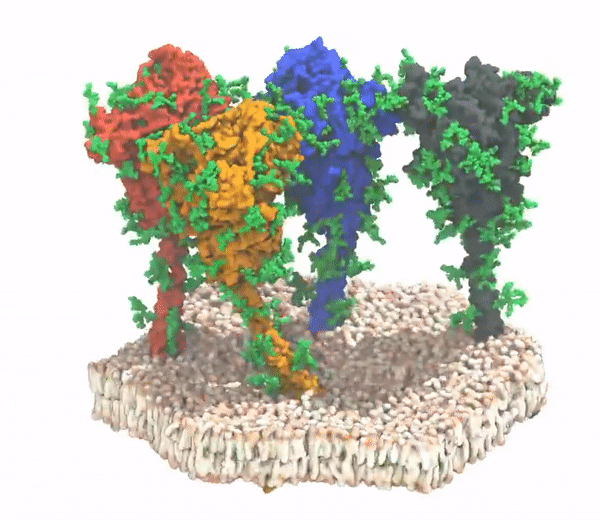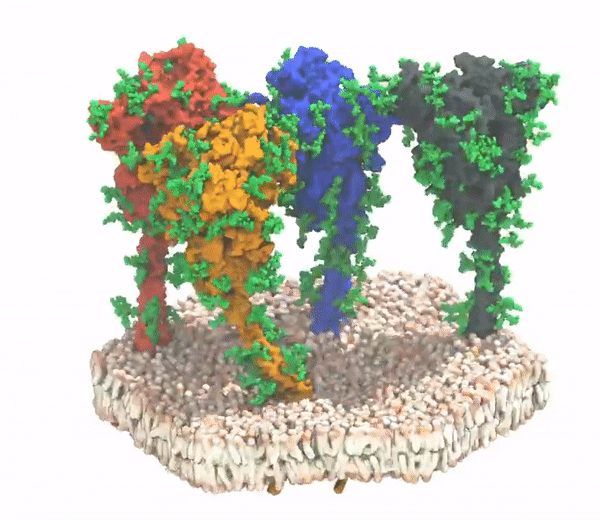
Me and my spikey bois when I invade a cell.
Graham (the NIH) had already been working with Moderna to create a vaccine against a different bat virus, Nipah, as a dress rehearsal for a real pandemic Also, McLellan’s lab licensed an even more potent version of their spike protein, royalty-free, to be incorporated into a vaccine for low- and middle-income countries. On 13 January, Moderna translated the stabilised spike protein into their mRNA delivery platform.
mRNA technology
The Pfizer and Moderna vaccines demonstrate the coming of age of nano-medicine. The mRNA vaccine field is developing rapidly as a large body of preclinical data has accumulated over the past several years, and multiple human clinical trials have begun.
Conventional vaccines usually contain inactivated pathogen (the disease-causing organism) or antigen (pathogen-specific protein), which stimulates the body’s immune system to develop antibodies, priming it for a faster and more effective response when exposed to the actual pathogen in the future.
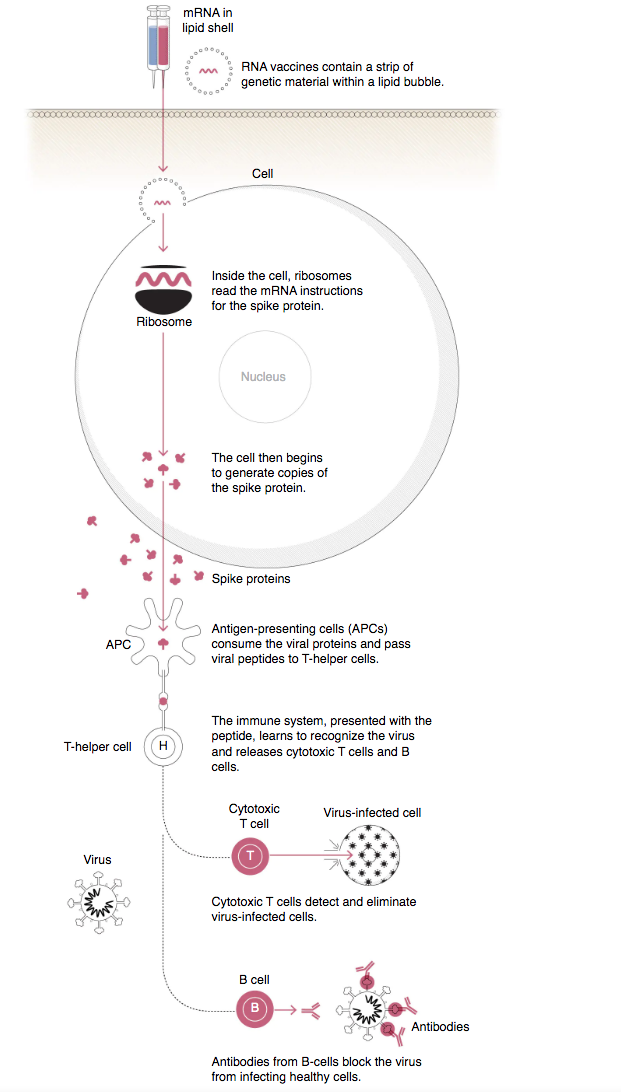
Human cells use DNA as the template to make mRNA, which are translated to build proteins. mRNA vaccines contain synthetic mRNA strand that codes for an antigen specific to the pathogen, which makes the host cell produce and display the antigen on its surface, where it is recognised by the immune system to produce antibodies.
All of the current vaccine candidates are designed for the spike protein of the D614 variant of the SARS-CoV-2 virus.
The details of mRNA technologies used by Moderna and BioNTech are undoubtedly proprietary (which accounts for the difference in storage temperatures), but they have to overcome the same obstacle: even before mRNA reaches its target cells, the body is very effective in identifying them as foreign and destroying them.
 I mean DNA is literally RNA X2
I mean DNA is literally RNA X2
The two companies’ solutions are more or less the same: wrap up the vulnerable mRNA strand in a nanoscale shell made of two layers of phospholipid analogous to that found in cell membranes, and add to that cholesterol which makes the bilayer more stable and less permeable. Add to the shell a lipid attaching a short chain of water-soluble polymer, which gives the nanoparticle a hairy coat to repel immune cells. Then add a tertiary amine head group that is an ionisable lipid (a weak base like ammonia) that can accept a proton to become positively charged, hence the charge of the nanoparticle depends on the pH of the environment.
By dissolving the four components of the shell in ethanol, and the mRNA strand in a mildly acidic solution, the nanoparticle will self-assemble. The ionisable lipid becomes positively charged when it contacts the acid, and since the RNA is negatively charged, the two start to bond. Other lipids self-organise into a bilayer sphere with hydrophilic heads on the outside, and hydrophobic tails on the inside, encapsulating the RNA inside. When the nanoparticle comes into contact with the human cell membrane, a negatively charged lipid on the membrane binds to the positively charged heads of the ionisable lipids in the nanoparticle, allowing entry of the mRNA strand into the cell.
One of the major advantages of mRNA vaccines is that mRNA is that there is no potential risk of infection or mutation. mRNA’s inherent tendency to stimulate immune response can also be down-modulated to increase safety. Another advantage is that mRNA is degraded by normal cellular processes, so its in vivo half-life can be modified. Efficient in vivo delivery also allows rapid uptake and expression in cytoplasm. mRNA is the minimal genetic vector, so unlike conventional vaccines, mRNA vaccines can be administered repeatedly without worrying about immunity against the vector. Lastly, mRNA vaccines have the potential for rapid, inexpensive and scalable manufacturing (see section IV.), mainly owing to the high yields of in vitro transcription reactions using readily available raw chemical products, compared to conventional vaccines that rely on cell culture in chicken eggs or mammalian cells.
The Pfizer and Moderna vaccines were definitely not overnight successes; they were built on more than a decade of hard work. The majority of early work in mRNA vaccines focused on cancer immunotherapy. In fact, BioNTech’s Şahin received substantial funding from the European Research Council to develop personalised cancer vaccines. Even shortly before the pandemic, mRNA vaccines had been experiencing a burst in basic and clinical research, with a number of papers demonstrated protection against a wide variety of pathogens, including influenza virus, Ebola virus, Zika virus, Streptococcus spp. and T. gondii. In fact, The US Biomedical Advanced Research and Development Authority (BARDA) has been funding Moderna’s clinical trial of a promising nucleoside-modified mRNA vaccine for Zika virus.
Genome sequencing
Rapid genome sequencing is one of the factors that allowed Moderna to produce its candidate in just 2 days in January. You can check out the source code of the Pfizer vaccine for yourself. The plummet in the cost of human whole genome sequencing (WGS) has even outpaced the Carlson curve6, which predicts that the doubling of DNA sequencing technologies as measured by cost7 and performance would be at least as fast as Moore’s law.
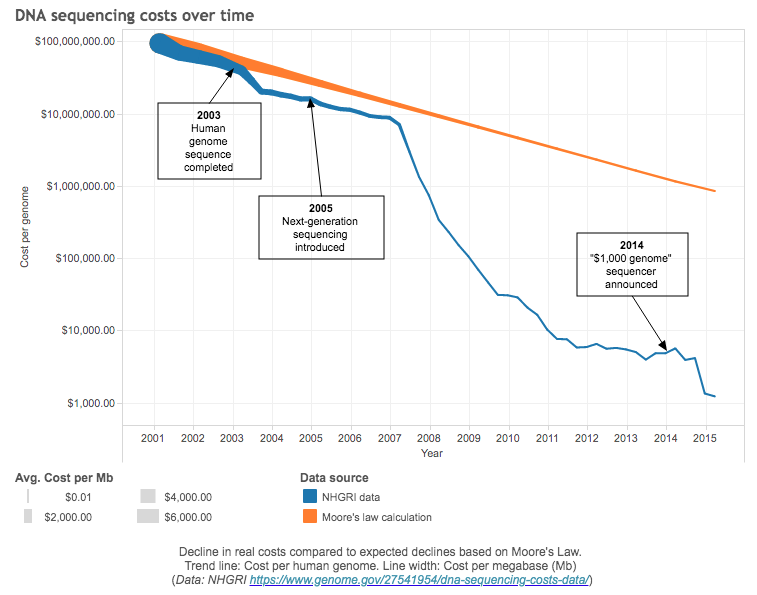 The pace of innovation in genome sequencing in the last decade has been astonishing.
The pace of innovation in genome sequencing in the last decade has been astonishing.
It took $5 billion (adjusted for inflation) and almost 15 years for the Human Genome Project to sequence the first human genome. In 2016, Veritas Genetics began selling WGS for $999, breaking the commonly-referred commercial target of $1000. Last year, Veritas Genetics cut the cost for WGS to $599. In February this year, since acquiring US’s Complete Genomics, China’s BGI8 claimed it could perform WGS for $100, which is the current commercial target.
SARS-CoV-2 is a positive-sense single-stranded RNA (ssRNA) virus. Positive-sense (5′-to-3′) means that the viral genome itself acts as mRNA that is directly translated by the host cell’s ribosomes into viral proteins, while negative-sense (3′-to-5′) means that it must be transcribed by an RNA-dependent RNA polymerase (RdRP) into a positive-sense mRNA, before being translated by the host cell’s ribosomes into viral proteins. And since human cells do not contain RdRP, positive-sense ssRNA virus will tell the host cell to make RdRP first in order to replicate the rest of its genome, while negative-sense ssRNA virus is packaged with RdRP in the virion (the entire virus particle) to first transcribe its genome into mRNA. In Cambridge, virus samples arrive the day after being taken, and are attached to an anonymised barcode. Once the sample is prepped, it gets pipetted into the injection port of the latest handheld minION sequencer, which can sequence the genomes of between 24 and 70 virus samples a day9.
Tracking SARS-CoV-2 outbreaks and sharing genome data10 globally is key in monitoring the pandemic. If a COVID ward has multiple samples of the same lineage, there might be a local outbreak within a particular area, community or perhaps even hospital.
Development
After sequencing the SARS-CoV-2 genome, Pfizer, Moderna and all the other biotechs could begin to develop vaccines11 and run clinical trials, which can normally take decades without any guarantee of success.
To develop a new vaccine, a highly variable amount of time is needed to find a candidate that will get past the clinical trials, and another 6.3 years are necessary to get past clinical trials and registration. If you are a clinical trial doctor, you normally spend most of your time submitting funding requests, resubmitting them, waiting, then submitting them somewhere.Then you have to renegotiate and wait for ethics approval. It is inevitable that there are problems with patient recruitment. After wading through more regulatory issues, you might have a therapy. At that point the company may not have deemed the therapeutic to be profitable, or NICE thinks it just is not worth paying for. Even after a therapeutic hits the market, it can be forced to withdraw12.
After passing clinical trials, it takes about 5-10 years to scale and distribute the vaccine. Even only looking at a vaccine candidate that is successful at every stage, it takes an average of ten years to develop from preclinical stages to launch, and an average of 7.6 years to go from Phase I to launch. Only 22% of developed vaccines are successful from start to finish, which means that 4.5 vaccine candidates are needed on average to produce a vaccine that works.
Looking back at the 27 most notable vaccines13, it gives a mean of 31.8 years of development, with a median of 27 years and a standard deviation of 17.7 years. If you exclude the vaccines still under development (e.g. HIV, malaria), the mean is 29.5 years (median 26, SD 17.4).
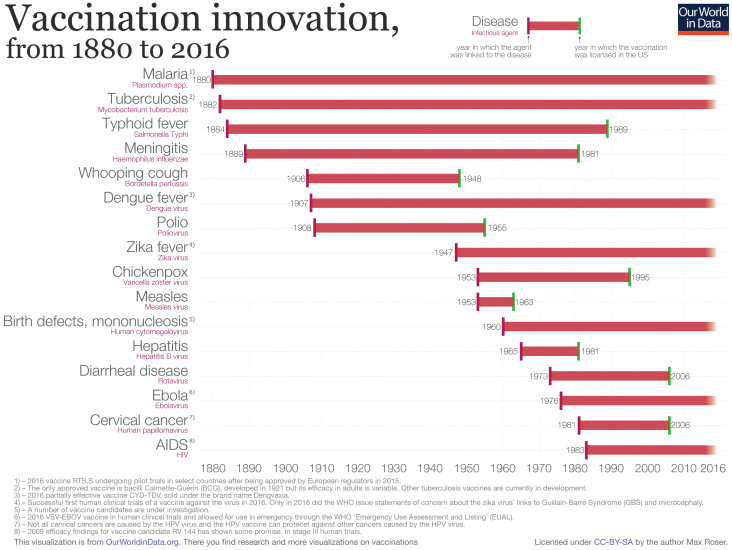 Unfortunately there are no vaccines for a number of pathogens despite decades of research.
Unfortunately there are no vaccines for a number of pathogens despite decades of research.
Until the end of 2020, there have never been any vaccine to prevent human coronavirus infections and treatment has only ever been supportive, even for the common cold, SARS-CoV-1 responsible for the 2002-2004 SARS outbreak, or MERS-CoV responsible for the ongoing MERS outbreak that started in 2012. One reason is the lack of funding: take MERS for instance, once the regional epidemic has been controlled, it meant demand for a vaccine stayed low. Another reason is that vaccines have bad business models: they require massive R&D budgets from preclinical studies all the way to completing the clinical trials, and if the vaccine provides long-lasting immunity, the companies can only sell so few vials. It is also just plain hard because it is difficult to tell what immune responses to coronaviruses are pathologic rather than protective, and there is a huge number of strains that mutate very rapidly (e.g. common cold). Vaccines are available for coronaviruses in chicken, pigs, and dogs, although their effectiveness is limited due to rapid mutation. In the case of outbreaks of highly contagious animal coronaviruses, entire herds of farm animals are destroyed.
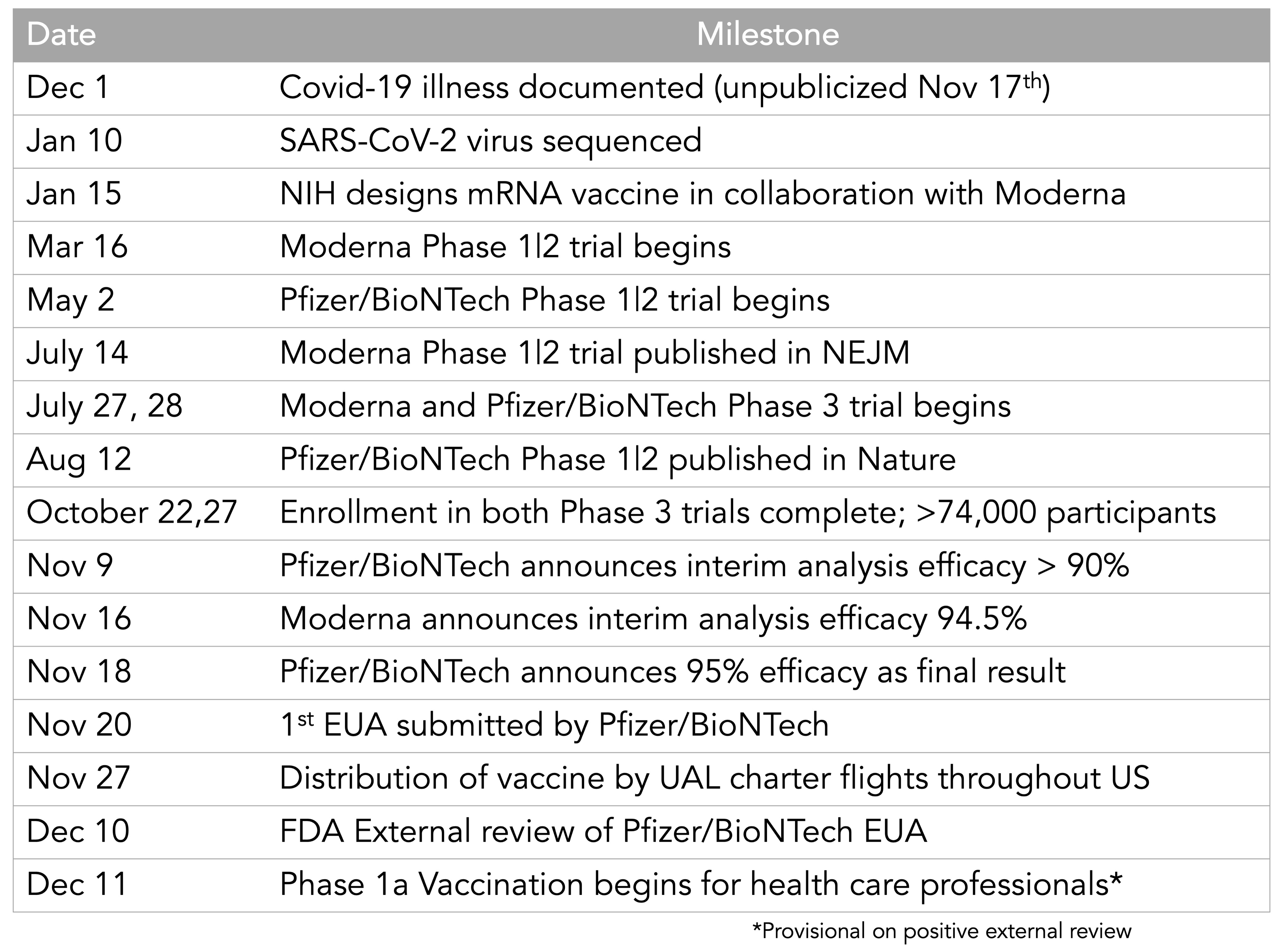
It is all the more amazing that the candidates have been developed so rapidly. On January 10 2020, the SARS-CoV-2 genome was published. Just 3 days later, Moderna finalised the sequence for their mRNA vaccine candidate; the first batch was manufactured on February 7. On February 24 (45 days after genome publication), Moderna shipped the first batch of mRNA-1273 to the NIH for use in their phase 1 clinical trial, followed by 266 days of clinical trials and regulatory coordination. On November 16, Moderna announced that the vaccine’s efficacy was 94.5%.
In the US, Operation Warp Speed (OWS), headed by Moroccan immigrant and molecular biologist Moncef Slaoui, was established with the goal of producing and delivering 300 million vaccine doses by January 2021. OWS purchased vaccines before clinical trials have been completed e.g. $1.6 billion for 100 million doses from Novavax, and $1.95 billion for 100 million doses from Pfizer. Funding agencies also directly invest in vaccine development. Governments and foundations like the Coalition for Epidemic Preparedness Innovations are throwing millions, even billions into the hunt for vaccines. Moderna14, for instance,received $472 million from BARDA in July, on top of the $483 million it got in April.
During the 2014-2016 West African Ebola crisis (the worst Ebola outbreak in history), regulatory bodies embraced a new dexterity in green-lighting trials and were a lot more proactive in communicating with companies about the efficacy thresholds. For COVID-19, the FDA has outlined that vaccines need to prevent infections or reduce the severity of COVID-19 in just 50% of recipients to be approved. Some of the trials have even been collapsed into Phase 1/2 or Phase 2/3 trials, which can shave weeks or even months off the trials. The Medicines and Healthcare products Regulatory Agency in the UK has been conducting rolling reviews of the Oxford vaccine. Similarly, the European Medical Agency has also been conducting rolling reviews of Moderna’s vaccine since 17 November.
Manufacturing
Even when the vaccines pass phase 3 trials, manufacturing is a long and arduous process15 with a myriad of stringent quality control measures at every step in the pipeline16. Not to mention the fact that all the later-stage candidates are based on completely novel or immature technologies that have never been scaled.
For the Oxford and Sputnik V vaccines, which are modified adenaviruses, the only therapeutic that uses a similar technology is gene therapy, which has only been used on single-digit thousands of patients (the Ebola vaccine is also a viral vector but it uses the VZV virus). Luckily, the UK already has four million doses of the Oxford vaccine ready to deploy as production has begun long before data from the Oxford phase 3 trials were analysed.
Conventional vaccine supply chains contain some unusual supply chains. Endotoxins shed by bacteria are one source of contamination, and to detect them, each batch of vaccine is tested with Limulus amebocyte lysate (LAL), of which the only natural source is horseshoe crab blood. Luckily, a synthetic version of LAL has recently been developed and approved by the FDA and the European health ministry, but scaling its production is still going to be challenging.
Many Covid-19 candidates require adjuvants e.g. QS-21, which in theory can shrink the volume of vaccine needed in each dose, easing the pressure on manufacturers. The active ingredient in QS-21 comes from the soapbark tree that grows in the mountains of Chile. Its bark has to be harvested in the southern summer, turned into a slurry, and processed. A synthetic QS-21 made from fermenting raw sugarcane in Brazil is still in development. Squalene, another new adjuvant, is derived from shark liver oil. To obtain 1 billion doses would take a lot of sharks, a lot of hunting, and a lot of pissing off GreenPeace.
Although the mRNA vaccines are the most advanced candidates they ironically have the least developed supply chain. Pfizer claims to eventually have enough capacity to produce 1.3 billion doses in 2021, which sounds like a lot but the vaccine requires 2 doses to work (and so do most if not all of the candidates). We need to vaccinate billions. It also does not help that Moderna will be calling the same suppliers for the raw material, all of which can be obtained as synthesised chemicals or bacterially expressed, animal component-free reagents, which allows mRNA vaccines to avoid safety concerns surrounding the unintentionally introduced microorganisms that plague cell-culture-based vaccine manufacture.
As mentioned before, mRNA degradation has to be prevented, and that requires a very rare substance called vaccinia capping enzyme (VCE). Just over 10 pounds of VCE is enough to produce 100 million doses of mRNA vaccine, but the current manufacturing processes require so much bioreactor capacity that it would cost $1.4 billion. More important, global bioreactor capacity cannot support production at that level while also producing other vaccines and cancer-fighting drugs.
Less exotic supply links such as needles and syringes will also be challenging to scale. The US Biomedical Advanced Research and Development Authority (BARDA) has invested heavily in a number of companies to make up for lost time. Schott AG, the world’s largest manufacturer of medical borosilicate, a special glass to make vaccine vials that can withstand the -80°C storage, will produce 2 billion vials following a $376 million investment. Corning Inc. received $204 million to expand its production of glass vials. ApiJect Systems Corp. has received $138 million to make 100 million pre-fillable plastic syringes by the end of the year. SiO2 Materials Science has received $143 million to produce 120 million patented vials made of plastic coated with a thin layer of glass, which will not be affected by a potential sand shortage.
It is not clear what the rate-limiting step(s) is in scaling up the Pfizer and Moderna vaccines immediately. Oddly, none of the official sources say what makes the scaling take months instead of weeks or years, even when many governments and Bill Gates are spending more than a billion dollars to accelerate it17. We have already seen the costs of supply-chain failures in the delayed production of simple nasal swabs that slowed testing by months even as cases exploded worldwide. Unfortunately, the delays with vaccine complementary infrastructure parts as Alex Tabarok has warned is very real and already causing Operation Warp Speed to underdeliver.
Distribution
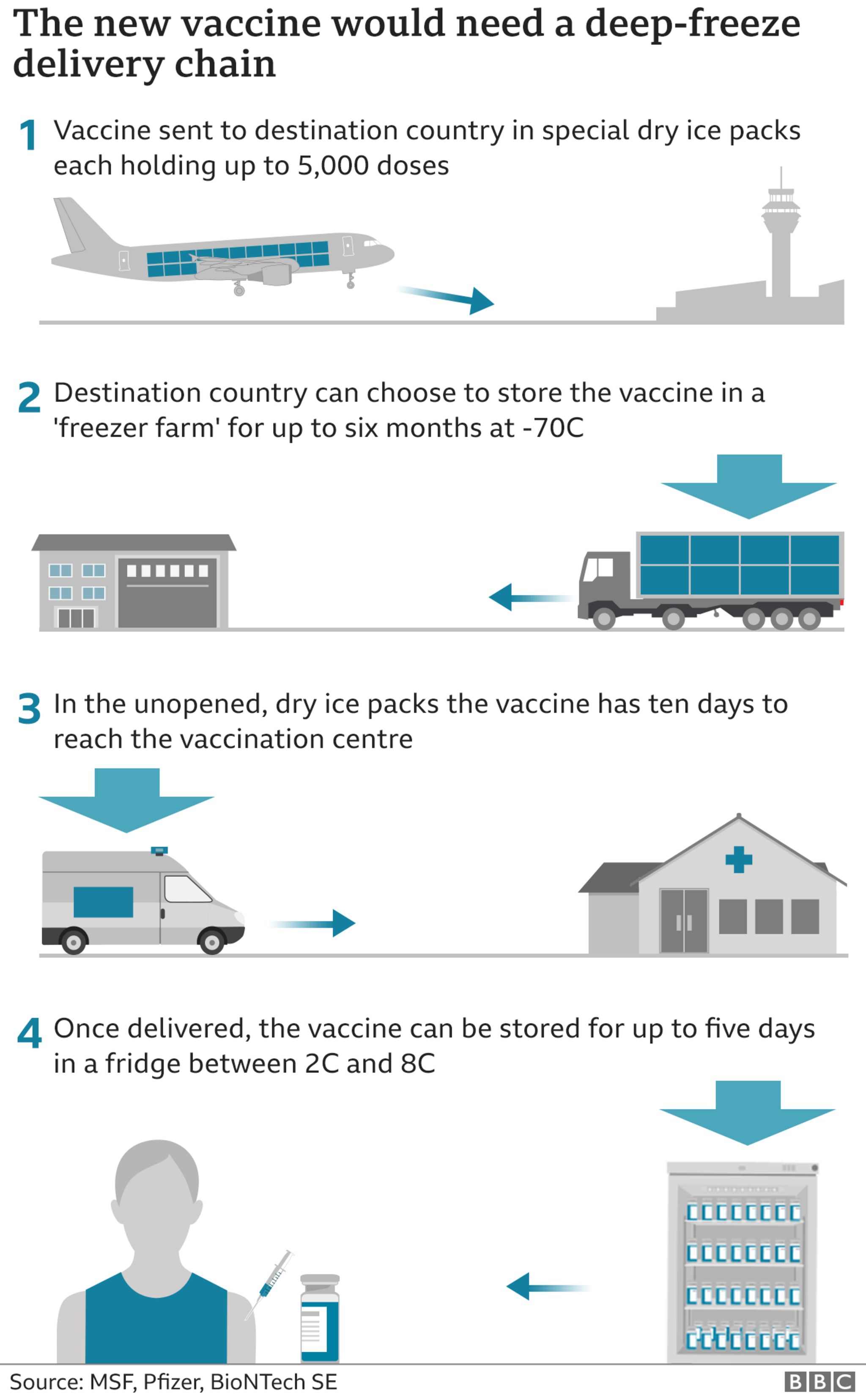
On top of manufacturing, distribution is going to be a tremendous logistical challenge, especially for the Pfizer vaccine that cannot be removed from -70°C more than 4 times. “Cold chain” delivery is hard enough for first-world cities, but to reach people living in rural parts of India, or sub-Saharan Africa, it will need new infrastructure.
Pfizer has designed a special box equipped with GPS-enabled thermal sensors, the size of a large cooler, that can hold a few hundred vials, each containing 10-20 doses of vaccine, but the boxes are not supposed to be opened more than twice a day and need to be closed within one minute of opening. Once it has thawed, the vaccine can be refrigerated for five days. UPS said it was constructing a “freezer farm” with 70 industrial freezers each capable of holding 48,000 vials in Kentucky. FedEx is also adding freezers that can maintain temperatures as low as -80°C in cities such as Memphis, Indianapolis and Paris. It is also installing additional refrigerated trailers in Oakland, Dallas and Los Angeles, which could be used for vaccines that need to be served chilled, not frozen.
All this freezing means the world is facing a looming shortage of dry ice, yet another unexpected side effect of the pandemic. Dry ice (made from carbon dioxide) is most commonly created as a byproduct during ethanol production, which declined as the demand for gasoline slumped during lockdowns.
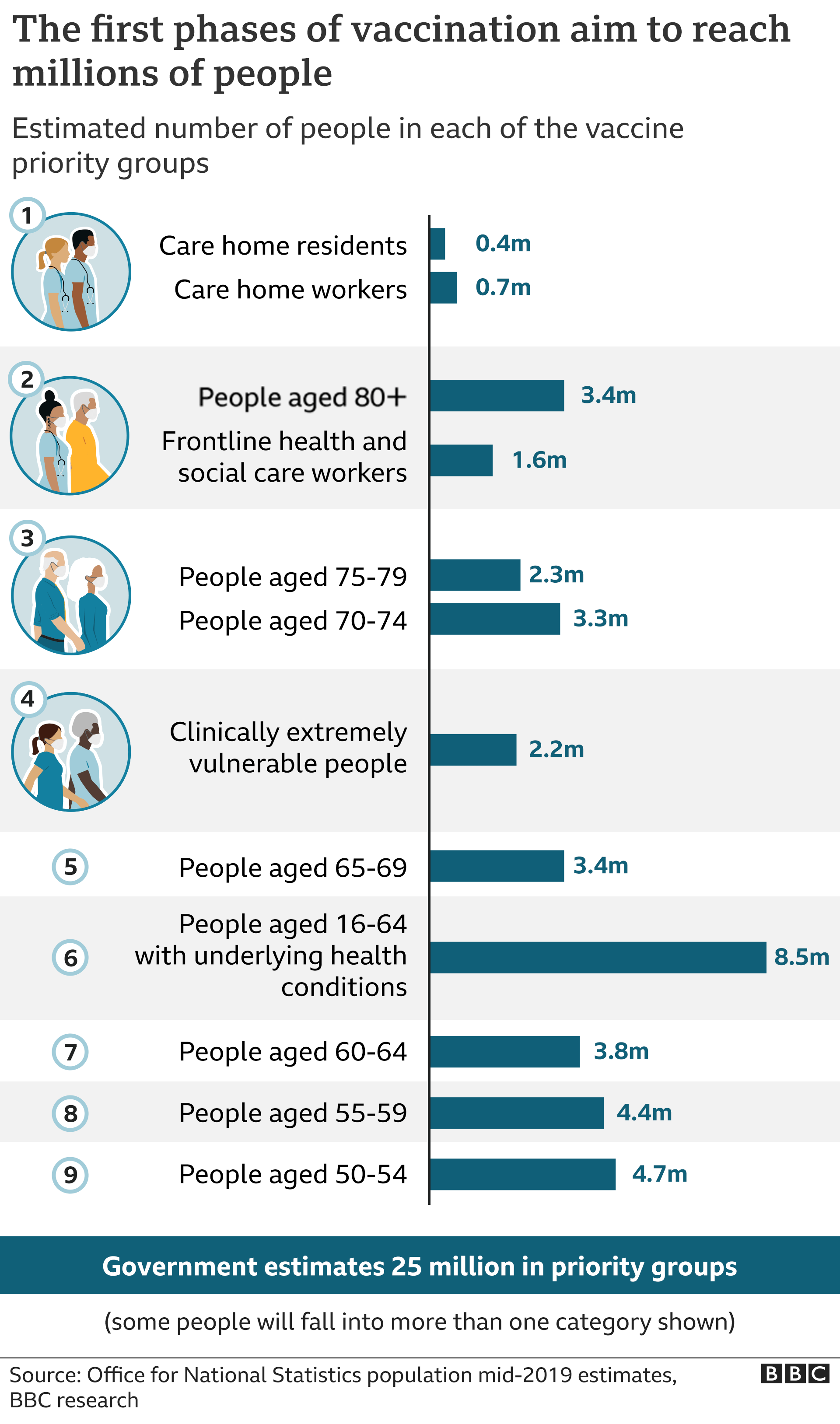
Then there is the problem of who will get the vaccine first. Emergency Use Authorisation (EUA) enables the staged entry of a vaccine, first for people at high risk e.g. health-care providers, first responders, the elderly, and those with high-risk comorbidities. Up next would be K-12 teachers, and critical workers in high-risk settings like public transit. The third phase includes young adults and children and other workers at increased risk, followed by everyone else in phase four.
[UPDATE 21/11/2020] The NHS plans to vaccinate all priority cohorts by the end of February, and England’s population by end of April. Its many assumptions include 75% uptake outside of residential settings (e.g. care homes and prisons) where 100% is expected, and the availability of more than 7 million doses in December. The plan also estimates 15-20% of vaccines wasted.
The dates of the rollout:
- Care home residents and staff, healthcare workers - from beginning of December;
- Ages 80 plus - from mid-December;
- Everyone aged 70-80 - from late December;
- Everyone aged 65-70 - from early January;
- All high and moderate risk under 65s - from early January;
- Everyone aged 50-65 - from mid January; and
- Everyone aged 18-50 - from late January; but with the bulk of this group vaccinated during March.
Time to buy Ryanair and AirBnB stocks!
[UPDATE 21/11/20] FDA just announced the meeting on Pfizer’s EUA to be held on 10 December18.
[UPDATE 29/11/20] The Pfizer vaccine could get UK approval as soon as December 7! Are they reckless? Meanwhile, the European Medicines Agency can only approve the various vaccines as soon as “the end of the year”. The Japanese are taking it even slower as they might require additional clinical trials within their country.
[UPDATE 02/12/20] The UK approved the PFizer/BioNTech vaccine!
[UPDATE 10/12/2020] The FDA advisory committee recommends EUA approval for the PFizer vaccine! (Votes against were for really stupid reasons)
[UPDATE 11/12/20] The FDA has issued EUA for the Pfizer vaccine!
[UPDATE 16/12/20] The UK has vaccinated more than 130,000 people in the first week since 8 December! Meanwhile, vaccination has just begun in the US on 14 December.
[UPDATE 18/12/20] The FDA has issued EUA for the Moderna vaccine!
Should we worry about anti-vaxxers and non-compliance? If surveys are worth anything, 1 in 6 respondents said they would refuse a vaccine. Another survey of 70000 people found that only half of the respondents are “very likely” to get vaccinated. A recent poll found that 58% Americans say they would be willing to get the jab, up from a low of 50% in September. We shall see. Maybe the hesitancy will take care of itself.
This does mean that initially the vaccines will not provide the sort of herd immunity that can help extinguish an epidemic, let alone a pandemic. EUA makes sense, but in the US alone, the National Academy of Sciences estimates that there are 16 million people working in health care, 2.1 million first-responders, and also 2.1 million elderly people living in nursing homes and other residential facilities. So even the first phase of rollout will require tough choices on distribution. Scaling manufacturing as best as we can is the antidote to distribution problems.
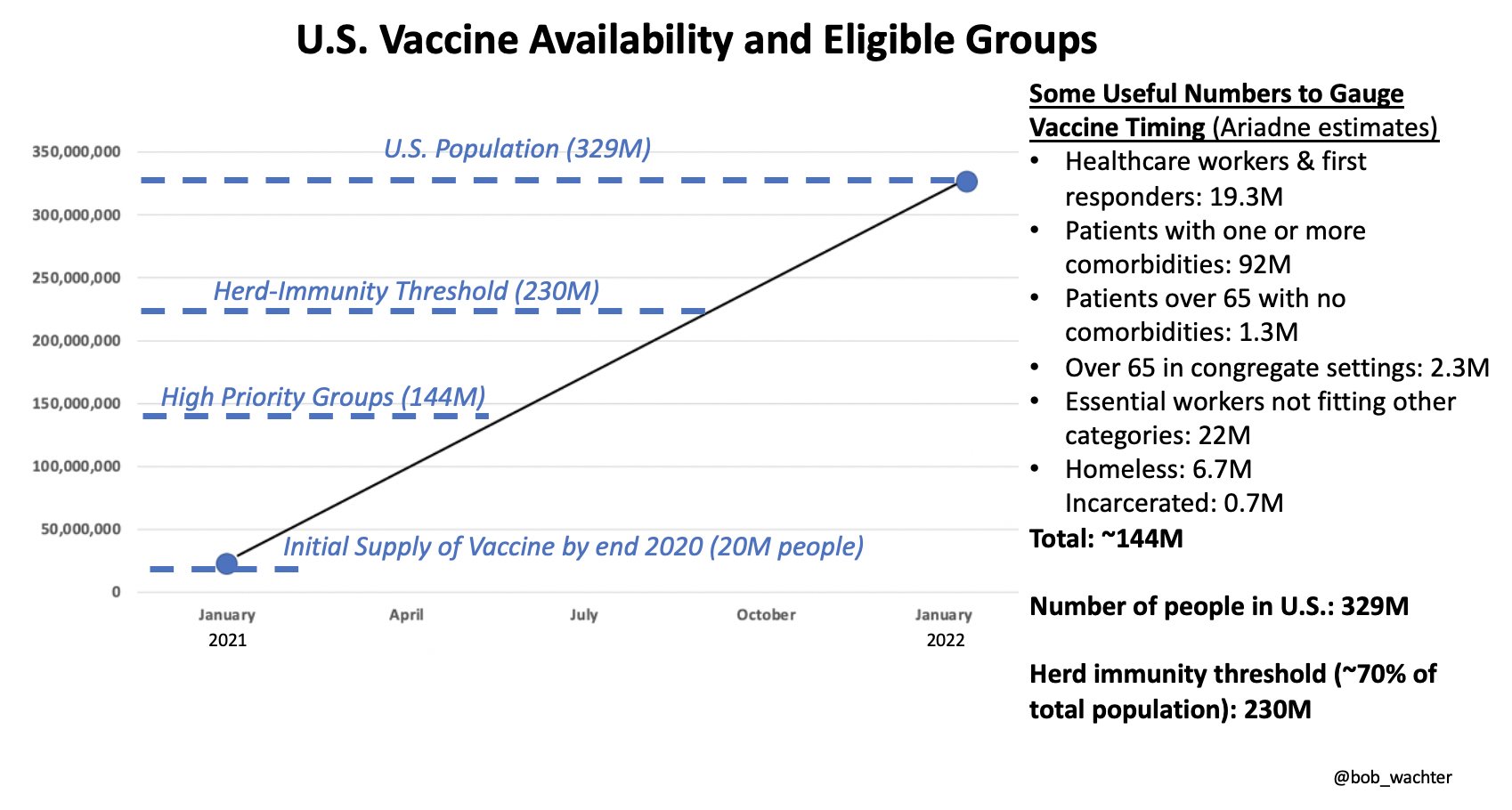 An estimate of US vaccine availability and eligible groups.
An estimate of US vaccine availability and eligible groups.
If there are only 4 million doses in the UK at the very first, hypothetically, it does not make sense to scatter them “fairly” among the 66-million population, as politics would suggest. There is a proposed way to ensure fair distribution: vaccine lottery, run by place (instead of individual), such as a whole hospital or a nursing home, weighted according to the number of inhabitants, which simplifies logistics and allows for more efficient data collection. Phase 3 trials typically compare 20000 people who got the vaccine with 20000 people who received a placebo, but statistical comparisons require waiting until enough people are exposed to the virus in their normal daily lives. Vaccine allocation by lottery will implicitly create a very large randomised trial. Lotteries are also reasonably straightforward to implement. The main issue would be to ensure that the lottery process is clear and transparent, and thus trustworthy.
Pfizer and Moderna both say that they can make in the range of 20 million doses by the end of the year, but what we do not know is (1) how many of these doses can be distributed and how it is going to happen, (2) what the number of doses available right now is, (3) how the ramp-up of both production and distribution are going to be coupled in the coming months, (4) what is going to show up with the other vaccine candidates in testing.
The very first people to get these new vaccines will almost surely be health care workers, starting some time in December. The rollout after that has too many variables to usefully predict, but it is going to be the biggest thing of its type ever attempted, in people-per-unit-time. The light is real, but the tunnel at the end of it is still pretty long. Only a tiny amount of people will receive vaccines when Santa comes to town19. In the meantime, if you have nothing better to do for the holidays, maybe sell your Zoom stock-ings and buy some Moderna20 ones so you can buy the vaccines from the blackmarket.
Endnotes
-
The development timeline is very impressive. Is the Great Stagnation coming to an end?
-
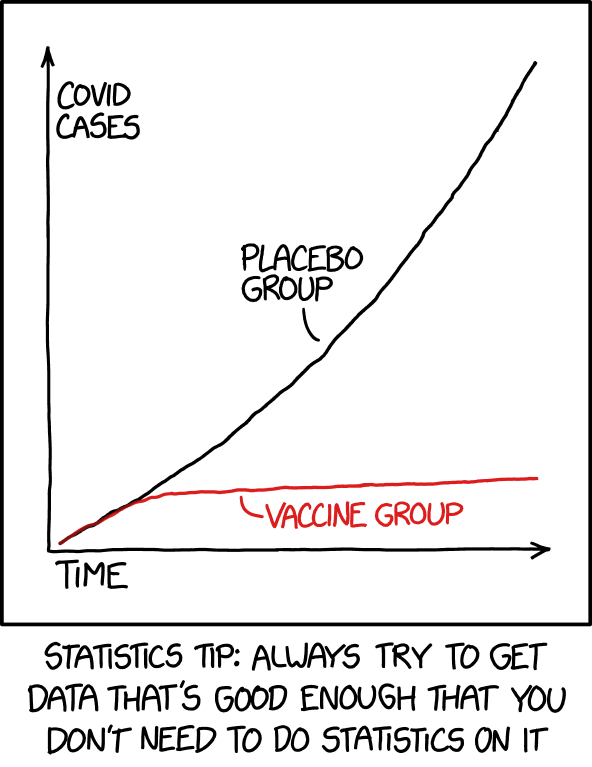
“We reject the null hypothesis based on the ‘hot damn, check out this chart’ test.” ↩
-
Viral vector vaccines have the advantage of setting off a full range of immunity responses because they contain actual viruses (weakened so they do not replicate). A disadvantage is that it has only once been used in human therapy (the Ebola vaccine), and regular adenovirus infects people anyway so some of the patients may already have antibodies to that one. It also means that booster shots would have an uphill battle. Antibodies to the viral payload? Good. Antibodies to the viral vector itself? Not good. ↩
-
Participants in the half-dose “arm” of the Oxford trial experienced less severe side effects, they might not have been able to deduce the arm they are in as well, hence better blinding than the full-dose arm. This could mean the efficacy of the full-dose protocol is higher than 62%! Note that the half-dose regimen was not tested on anyone over 55, unfortunately. As Bob Ross said, “We don’t make mistakes, just happy little accidents.” ↩
-
Although Moderna has made each of the founders boatloads of amounts of money, even before the company had produced a single product, Rossi accused Langer and Afeyan of propagating a condescending myth that he did not understand his discovery’s full potential until they pointed it out to him. Fighting for credit in academia is a tale as old as time. ↩
-
The Carlson curve started being profoundly outpaced in 2008 due to maturing next-gen sequencing from Illumina (definitely not the Illuminati), which acquired UK startup Solexa and its gigabase-level sequencing technology in 2007. It remains an open question as to what happened around late 2012, when the exponential price decrease was halted for years, keeping whole-genome sequencing prices high, and thus delaying whole-genome sequencing replacing SNP genotyping in genetics research by up to a decade (with untold medical consequences). Was it just the Illuminati’s fault and a failure of anti-trust law? ↩
-
Bacterial genomes are on average 600 times smaller than a human genome, so the costs with sequencing are significantly lower. However, only 15% of the cost of sequencing a bacterial genome goes to the process of gathering the nucleic bases from the sequencing machine. For a human genome, it is closer to 70%, because it takes many more reads to achieve a working level of sequence coverage for a 3-billion-base-pair human genome than a bacterial one, but sample preparation and library construction costs do not necessarily scale with genome size or read coverage. ↩
-
In 2003, to investigate the genomics of intelligence, BGI launched a cognitive genomics lab in Hong Kong where more than 100 gene-sequencing machines deciphered 2,200 genomes, the majority of which were from people with IQs of 160 or higher, supplied by a researcher at King’s College London, and the Vice President was Stephen Hsu. ↩
-
Computing power and storage capacity are nonetheless bottlenecks because sequencing generates a lot of raw data. The 1000 Genomes project data, for example, consists of 200 terabytes (1 terabyte is 1,024 gigabytes) for the 1700 participants in 2014.
The first SARS-CoV-2 strain sequenced on 18 March 2020: Wuhan-Hu-1, has 29,903 nucleotides. Since there are only four possible nucleotides, we can estimate the information compression value of each nucleotide at approximately 2 bits. Therefore, in theory, the Wuhan-Hu-1 genome requires only 7.5 kilobytes to store. ↩
-
There is still room for GWS to improve. The Netherlands, for example, has been using an amplicon-based sequencing approach, which relies on close, reliable reference sequences that are designed based on current knowledge about SARS-CoV-2 diversity and therefore need regular updating. However, at the moment, conventional metagenomic sequencing from Illumina takes too long for near to real-time sequencing, and nanopore-based metagenomic sequencing is not sensitive enough to allow recovery of whole-genome sequences in a similar fashion and costs compared to amplicon-based nanopore sequencing. In the future, this may be overcome using metagenomic sequencing. ↩
-
An early form of vaccination was called “variolation” or “inoculation”. The oldest practice was an ancient Asian technique of blowing dried smallpox scabs up the nose to infect the person with a milder form of the disease. By the 1700s, variolation had spread to Africa, India and the Ottoman Empire, followed by the UK and America, where skin puncture was more frequently performed. In 1796 English physician Edward Jenner observed that an infection with cowpox can protect against smallpox. In a move that will make current-day ethics boards think you are Literally Hitler, Jenner used matter from a cowpox lesion to inoculate his gardener’s 8-year-old son, who was then exposed to smallpox lesion matter after 2 months. He did not develop smallpox so Jenner concluded that the wee child was protected against the disease, and coined the procedure as “vaccination”. ↩
-
The Lyme vaccine LYMErix™ serves as a cautionary tale on risk communication by physicians and mass media, which can pre-empt clinical trials. In 1998, the FDA approved the new recombinant Lyme vaccine, which reduced new infections in adults by 80%. Just 3 years later, the manufacturer voluntarily withdrew its product from the market amidst falling sales, extensive media coverage of “vaccine victims”, and ongoing litigation, even though studies show that it was a cost-effective public health intervention for high-risk patients. The withdrawal represented a loss of a powerful tool for Lyme disease prevention. ↩
-
The list of the “most notable” 27 vaccines is definitely not exhaustive, it is just from WHO’s list of essential vaccines. ↩
-
Dolly Parton donated $1 million to COVID-19 vaccine research, which supported the development of the Moderna vaccine! ↩
-
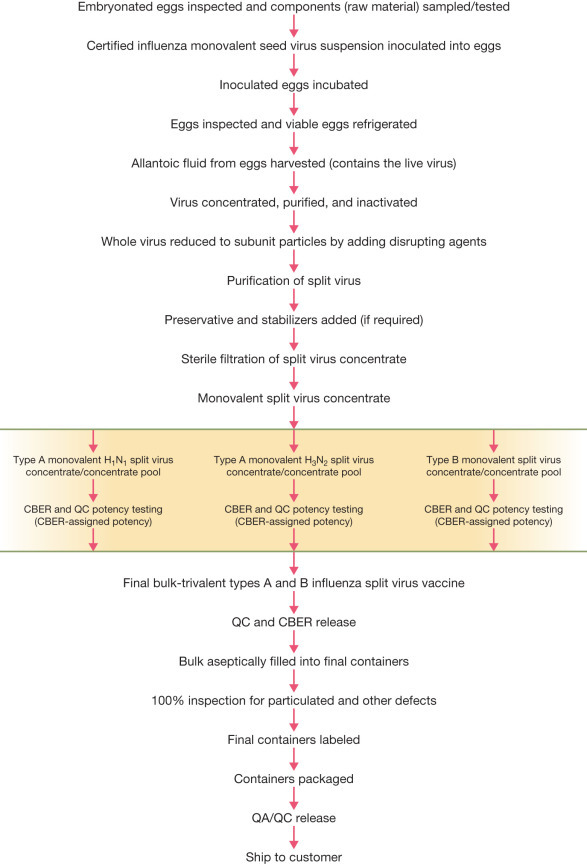
The first step in conventional vaccine manufacturing is the establishment of a “master cell bank”: a collection of vialed cells that form the starting material for all future production. Viruses are grown in either primary cells (e.g. chicken fibroblasts for yellow fever vaccine), or continuous cell lines (e.g. MRC-5 for hepatitis A vaccine). Bacterial pathogens are grown in bioreactors using media carefully optimised for the yield of the antigen while maintaining its integrity. Recombinant proteins can be manufactured in bacteria, yeast, or cell cultures. The next step is to isolate the antigen from the growth medium. This can be isolation of free virus, proteins from cells, or cells containing the antigen. The antigens are then purified using column chromatography, ultrafiltration, or simple inactivation of the isolated virus.
The formulation of the vaccine is designed to maximise the stability, distribution efficiency, and proper delivery. The formulated vaccine may include an adjuvant to enhance the immune response, stabilisers to prolong shelf life, and preservatives to allow multi-dose vials to be delivered. All components that constitute the final vaccine are uniformly mixed in a single vessel. Individual, scrupulously cleaned, depyrogenated, single or multi-dose containers are then filled with the vaccine and sealed with sterile stoppers or plungers. ↩
-
Take influenza as an example to see how complex quality control is.
-
COVID-19 Vaccines Advance Market Commitment (COVAX AMC) aims to ensure that the 92 middle- and lower-income countries will receive enough doses to vaccinate up to 20% of their population in the longer term. It is funded largely with public money and led by two global nonprofits that Bill Gates helped launch and bankroll, along with the World Health Organization.
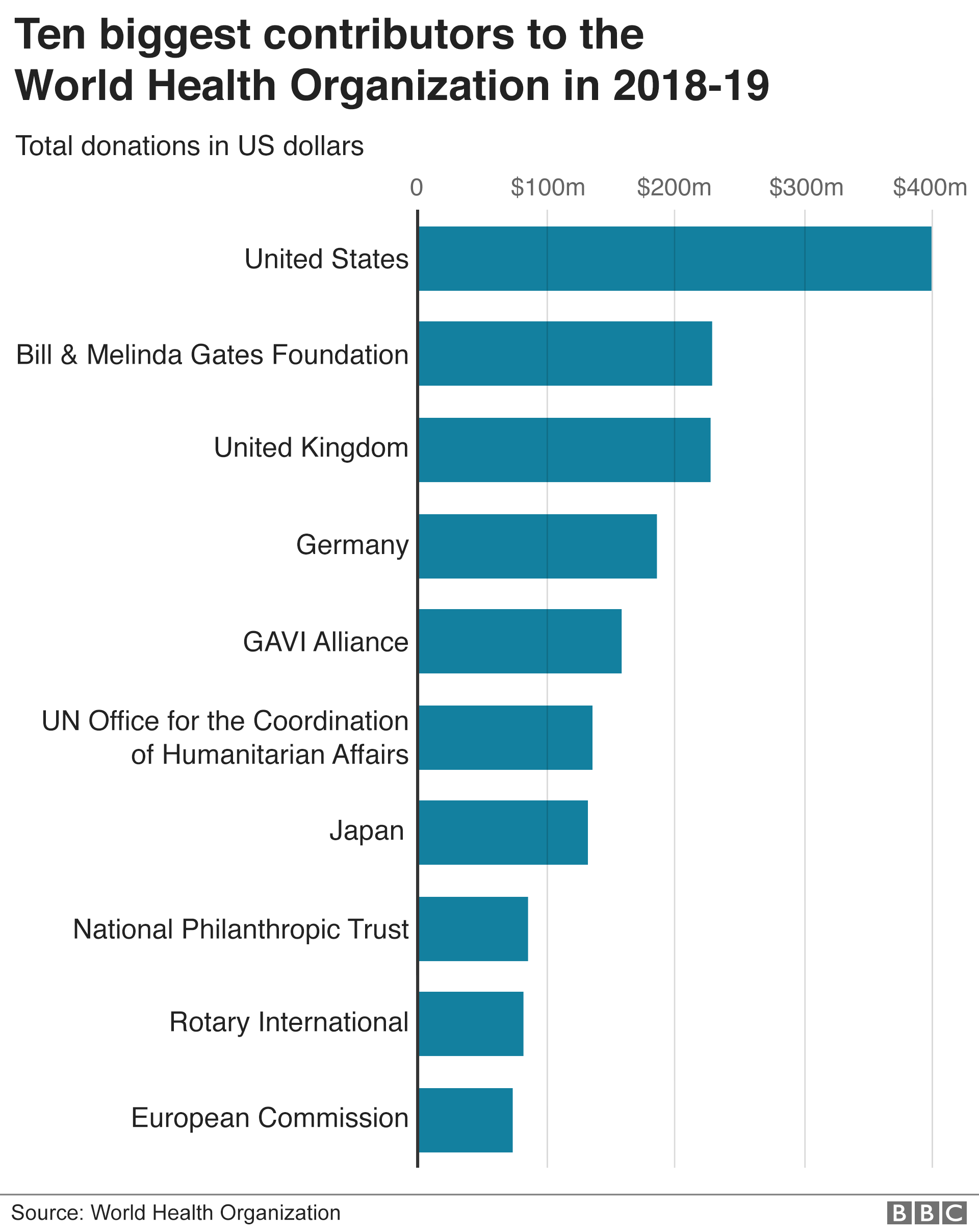 The Gates Foundation is the also the 2nd largest donor to the WHO, only below the US, higher than the UK and the rest of the world. ↩
The Gates Foundation is the also the 2nd largest donor to the WHO, only below the US, higher than the UK and the rest of the world. ↩ -
Why not sooner? The costs of approval delay are likely very high.
“FDA is required to publish announcements of advisory committee meetings at least 15 calendar days before a meeting date in the Federal Register (41 CFR sec 102-3.150). Once a meeting is published in the Federal Register, it is posted on FDA’s advisory committee meeting calendar, which can be found on the Advisory Committee Calendar page.” If they published today, and it probably takes at least a day or two to publish, it would be 18 days.
Even then, why take 3 weeks to review Pfizer’s application? Within 4 weeks, when the first wave in Europe was in full swing, BioNTech had identified 20 vaccine candidates as part its Project Lightspeed, so the FDA better be doing important stuff in the 3 weeks.
Are the 3 weeks warranted caution? In the 1976 swine flu epidemic in the US, a vaccine was rushed through with unexpected public side effects, causing less people to be willing to take the vaccine. Perhaps this is why the FDA is taking it slow. ↩
-
Santa would be the ultimate super-spreader responsible for more deaths than the bubonic plague. Luckily, Dr Anthony Fauci said the obese, old man has good innate immunity to COVID-19 (must be the Santabodies), so he can solve the distribution problem with his reindeers! But even the North Pole is 30°C too hot for the Pfizer vaccine. Santa’s child labourers should just extract his serum to massively scale production, with proper ‘elf and safety measures of course.
Unless Santa’s sweatshop can produce a Christmas miracle, the holidays are going to be grim.
Note that contrary to the pernicious myth that those who die of COVID-19 are at death’s door anyway, the virus takes away 10 years of their lives. Luckily, as far as we know, Santa is immortal. ↩
-
Moderna’s Chief Medical Officer Tal Zaks has been getting $1 million richer each week by selling his existing stock like clockwork through pre-scheduled trades every week. As of early October 2020, it has earned him more than $50 million since the dawn of the pandemic.
Yeah, yeah, I know. But if vaccine makers cannot capture a tiny sliver of the billions or trillions of dollars in the value they bring, maybe no one should be allowed to be a millionaire! ↩